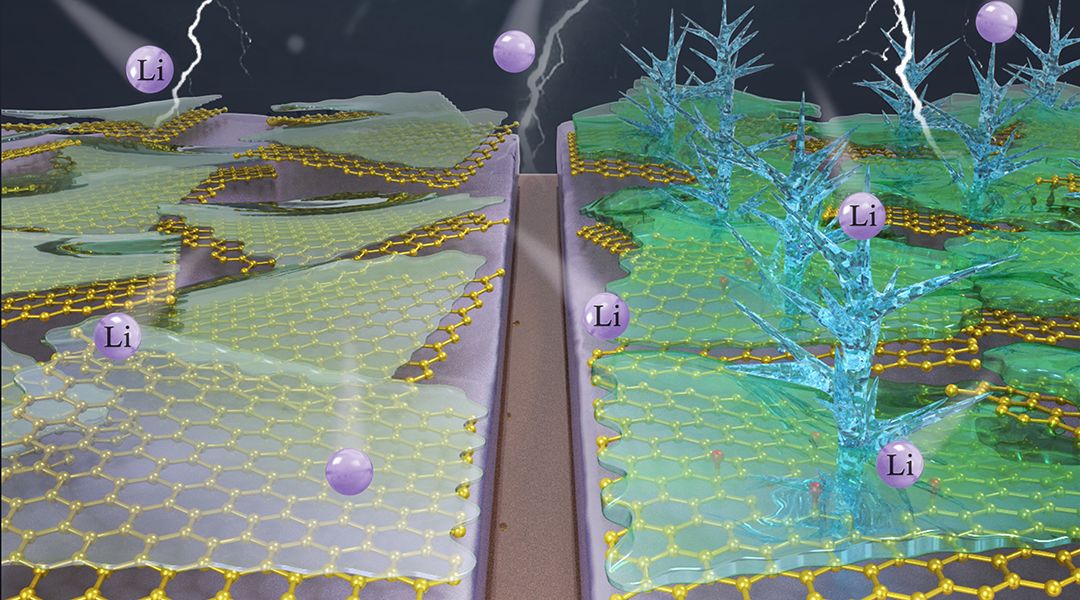
Lithium (Li) metal batteries (LMBs) are promising alternatives to Li-ion batteries (LIBs) given the much higher energy density associated with the lithium metal anode—the “Holy Grail” of electrodes. Energy-intensive applications that require longer operation times, like electric vehicles and drones, would take off if LMBs reach large-scale commercialization.
This promise, however, is tainted by the formation of a solid electrolyte interphase (SEI) layer on metallic Li in conventional carbonate electrolytes, which occurs as a result of irreversible reduction and chemical decomposition of the electrolyte in contact with the anode.
The SEI layer is associated with the growth of Li dendrites, leading to short cycle life and potential safety concerns. In order to address this critical issue, several strategies have been employed, including the use of electrolyte additives and carbon-based supports (e.g., graphene, graphene oxide, and nitrogen-doped graphene) to stabilize the Li metal anode.
A clear understanding of how the structure and chemistry of the carbon host affects Li dendrite growth and battery cell performance has been lacking until now. Recently, a team of researchers from Clarkson University (USA) and Sichuan University (China) has made a significant advancement in battery research by explaining how graphene defects play an important role in the formation of dendrites.
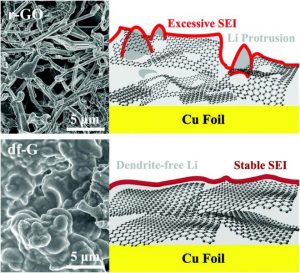 They compared intrinsic SEI formation on ultrapure, “defect-free” graphene (df-G), fabricated using a novel wet approach called “flow-aided sonication,” to a highly defective Hummers graphene (r-GO).
They compared intrinsic SEI formation on ultrapure, “defect-free” graphene (df-G), fabricated using a novel wet approach called “flow-aided sonication,” to a highly defective Hummers graphene (r-GO).
In the absence of electrolyte, the first cycle irreversible capacity loss of r-GO reached 1863 mA g−1 compared to 148 mAh g−1 for df-G. When fluoroethylene carbonate (FEC) was added, df-G yields state-of-the-art electrochemical performance with a relatively smooth, dendrite-free surface morphology.
In contrast, r-GO promotes rapid consumption of the FEC electrolyte, leading to extensive dendrite formation.
These observations uncover a critical new finding: SEI formation occurring before metal plating actually dictates dendrite formation. That is, the fate of the Li metal anode is effectively sealed once the carbon host forms an SEI at the initial charge.